Is Chemical Energy Converted to Electrical Energy in Electrolysis?
Yes, chemical energy is converted to electrical energy in electrolysis. This process happens when an electric current is passed through a liquid that contains ions. The ions are attracted to the electrodes, where they undergo a chemical reaction.
This reaction produces electricity, which can be used to power devices or does work.
In electrolysis, water is decomposed into its constituent elements, hydrogen, and oxygen, by passing an electric current through it. This process is used to produce pure hydrogen gas for use in fuel cells and other applications. The electric current passes through a container of water, called an electrolyte, which contains dissolved ions.
The ions are attracted to the electrodes (positive and negative poles) in the circuit and are drawn towards them. At the negative electrode (cathode), oxygen gas is produced. At the positive electrode (anode), hydrogen gas is produced.
The overall reaction can be represented as: 2H2O → O2 + 4H+ + 4e− This process requires energy to drive the reactions at the electrodes.
This energy comes from the electrical energy supplied by the battery or power supply in the circuit. In other words, chemical energy in the form of electricity is converted into chemical energy in the form of gases (hydrogen and oxygen).
How is the Chemical Energy in a Battery Converted to Electrical Energy?
In order to understand how a battery converts chemical energy into electrical energy, it is first important to understand what each of these terms means. Chemical energy is the potential energy that is stored in the bonds between atoms and molecules. This type of energy can be released through chemical reactions, such as when fossil fuel is burned.
Electrical energy, on the other hand, is the flow of electrons through a conductor, such as a metal wire. Now that we have defined these terms, we can better explain how a battery converts chemical energy into electrical energy. A battery consists of two electrodes (typically made from metals), separated by an electrolyte (a substance that conducts electricity).
When the battery is connected to an external circuit, ions in the electrolyte flow from one electrode to the other, creating an electric current. This movement of ions creates a potential difference between the two electrodes, which allows for electrons to flow through the external circuit and power whatever device is attached to it. The chemical reaction that occurs within a battery is known as an oxidation-reduction reaction (or redox reaction).
In this type of reaction, one element loses electrons while another element gains them. The loss and gain of electrons cause atoms to change their oxidation state; this change in oxidation state results in a release or absorption of energy. In a batteries case, the oxidation-reduction reaction releases enough energy to allow for electrical current to flow through an external circuit.
Chemical Energy Converted to Electrical Energy Examples
Converting chemical energy to electrical energy is a process that occurs in many different ways. One example is the use of batteries. Batteries are devices that store chemical energy and convert it to electrical energy when needed.
The most common type of battery is the lead-acid battery, which contains lead and acid electrolytes. When a lead-acid battery is discharged, the lead and acid react chemically to produce electricity. Other examples of converting chemical energy to electrical energy include fuel cells and solar cells.
Fuel cells are devices that use chemical reactions to generate electricity. Solar cells are devices that convert sunlight into electrical energy.
Chemical Energy is Converted Directly into Electrical Energy in Quizlet
Chemical energy is one of the most important forms of energy that powers our world. It is responsible for everything from the sun’s heat and light, to the electricity that powers our homes and businesses. In its simplest form, chemical energy is the energy that is released when a chemical reaction occurs.
This can be as simple as the burning of a candle, or as complex as the combustion of fossil fuels. In either case, chemical reactions involve the rearrangement of atoms and molecules, which releases energy in the form of heat and light. The most common way that we convert chemical energy into electrical energy is through batteries.
Batteries are devices that store chemical energy and then release it in a controlled way to power an electric current. The first battery was invented by Alessandro Volta in 1800, and today there are many different types of batteries available to meet a variety of needs. Batteries work by using a chemical reaction to create an electric potential difference between two electrodes (the positive and negative poles).
When electrons flow from the negative electrode to the positive electrode, they do work (in the form of moving an electric charge) which can be used to power electric devices such as lights or motors.
This Energy is Formed Upon the Conversion of Chemical Energy into Electrical Energy Or Vice Versa
If you’ve ever wondered how electricity is generated, you’re not alone. It’s a common question with a complicated answer. In short, electricity is created when energy from an outside source is converted into electrical energy.
This can happen in a number of ways, but most commonly it occurs through the conversion of chemical energy into electrical energy or vice versa. Let’s take a closer look at how this process works. When fossil fuels like coal and natural gas are burned, they release chemical energy.
That chemical energy can then be used to power generators that create electricity. Water can also be used as a source of renewable energy for generating electricity. When dams are built across rivers, the flowing water creates kinetic energy.
That kinetic energy can be harnessed and turned into electrical current using turbines and generators. So there you have it! Now you know that electricity is simply another form of energy that can be produced through various means of conversion.
Chemical Energy Definition
Chemical energy is the potential of a chemical substance to undergo a chemical reaction to produce other substances. This potential energy is stored in the bonds between atoms in the molecule. When these bonds are broken, chemical energy is released and can be used to do work.
The most common way that we use chemical energy is through combustion. In this process, a fuel reacts with oxygen from the air to release heat energy. This heat can then be used to power engines or generate electricity.
Fuels such as coal, oil, and natural gas are all sources of chemical energy. In living organisms, chemical reactions occur constantly to maintain life processes such as metabolism and cell division. These reactions require enzymes that act as catalysts to lower the activation energy required for the reaction to occur.
The food we eat contains nutrients that our cells use for fuel and this process releases chemical energy that we use to move, think, and breathe.
What is Electrolysis?
In chemistry and manufacturing, electrolysis is the process of using electricity to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential.
Examples of Chemical Energy
Chemical energy is the potential of a chemical substance to undergo a transformation through a chemical reaction or physical change. It is released during these processes, often in the form of heat or light. Common examples of chemical reactions that release energy are combustion (burning), decomposition, and oxidation.
When gasoline burns in an engine, the resulting exothermic reaction releases a great deal of energy in the form of heat and light. This energy can be harnessed to power vehicles, generators, and other machines. In decomposition reactions, molecules are broken down into simpler components.
This can occur naturally, as when organic matter decays or when food spoils. It can also be caused by man-made processes such as waste incineration or the breakdown of hazardous materials. These reactions usually release energy in the form of heat.
Oxidation reactions involve the loss of electrons from atoms or molecules. They can occur naturally, such as when iron rusts or when fruits and vegetables turn brown after being exposed to air. They can also be caused by man-made processes such as burning fossil fuels or smoking cigarettes.
Electrical Energy Definition
Electrical energy is a type of energy that results from the flow of electrons. It is a form of energy that can be converted into other forms, such as heat, light, and mechanical energy. Electrical energy is often used to power electronic devices, such as computers and cell phones.
FAQs
What Energy is Converted in Electrolysis?
In electrolysis, an electrical current is used to drive a chemical reaction that would not otherwise occur. The current passes through a solution containing ions ( electrically charged atoms or molecules) and causes one or more of the ions to be discharged (or oxidized) at the cathode (-), while the other ion is reduced (or deposited) at the anode (+). The overall effect of this process is the decomposition of water into hydrogen and oxygen:
2H2O → 2H2 + O2 The same general principles can be applied to other types of reactions, such as the reduction of metal oxides to metals:
Is Chemical Energy Converted to Electrical Energy?
In short, yes. Chemical energy can be converted to electrical energy. This is done by using a chemical reaction to create an electric current.
The most common way to do this is through the use of batteries. In a battery, there are two electrodes (metal plates) that are separated by an electrolyte (a liquid or gel that contains ions). When the battery is connected to a circuit, the chemicals in the electrolyte react with the metal plates, creating an electric current.
This current can then be used to power electrical devices. Other ways of converting chemical energy to electrical energy include fuel cells and solar cells. Fuel cells work by combining hydrogen and oxygen gases to produce water and electricity.
Solar cells convert sunlight into electricity using a process called the photovoltaic effect.
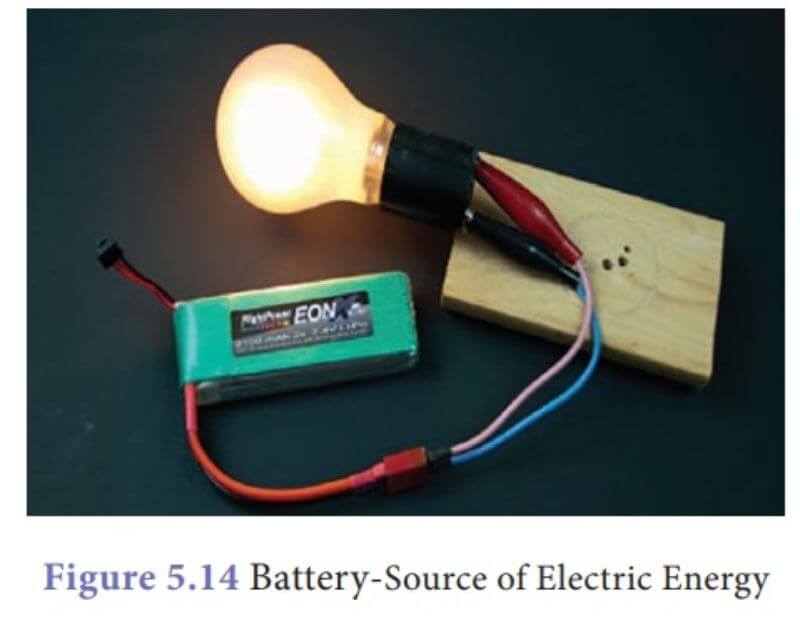
How is Chemical Energy Transformed Into Electrical Energy in a Battery?
In a battery, chemical energy is transformed into electrical energy. This occurs when the electrons flow from the negative terminal to the positive terminal. The chemical reaction that creates this flow of electrons is called an oxidation-reduction reaction.
In order for this reaction to occur, there must be a conductor between the two terminals. This conductor is typically made of metal, and it allows the electrons to flow freely between the two points.
Conclusion
In electrolysis, water is decomposed into its constituent elements of hydrogen and oxygen gas. This process requires an electrical current which provides the energy to split the water molecules apart. The electrical current is supplied by a power source such as a battery.
The chemical energy stored in the water molecules is converted into electrical energy which powers the electrolysis reaction.
Learn More:
- What is the Relationship Between Electric Power And Energy?
- How Long to Does It Take to Charge a 4.8 V Battery Pack?
Used Resources:
